R&D Center (Synthesis R&D Center / Bio R&D Center)


-
Sungwun Pharmacopia R&D Center
Sungwun Pharmacopia Synthesis/Bio R&D Center has been developing synthetic products focusing on intermediates, raw materials, electronic materials, cosmetics raw materials, and bio products such as fermented microorganisms, enzymes, and human-derived microorganisms. We have various networks and pipelines through collaboration with many domestic and foreign pharmaceutical companies. Based on this, we are aiming to become a global research company through collaboration with multinational pharmaceutical companies and licensing strategies by discovering and developing excellent tasks that require innovative products and advanced technology.
-
Improving the Differentiation and Flexibility through Separation and Concentration
Sungwun Pharmacopia's R&D Center is a differentiation strategy that separates and operates as a synthetic R&D Center and a bio R&D Center. We are studying biopharmaceuticals using existing chemical drugs and next-generation technologies at the same time, adding flexibility to technology development, and improving the possibility of success of innovative products and excellent product development through organic collaboration and efficient resource distribution in each research field.
-
Continuous investment in R&D
The R&D Center of Sungwun Pharmacopia has been investing in research and development since its establishment. We have a synthetic R&D Center in South Korea and Indonesia, respectively, to build and build a pipeline for technology development, and to optimize it for customer needs by region to increase the capacity for commissioned research and production.
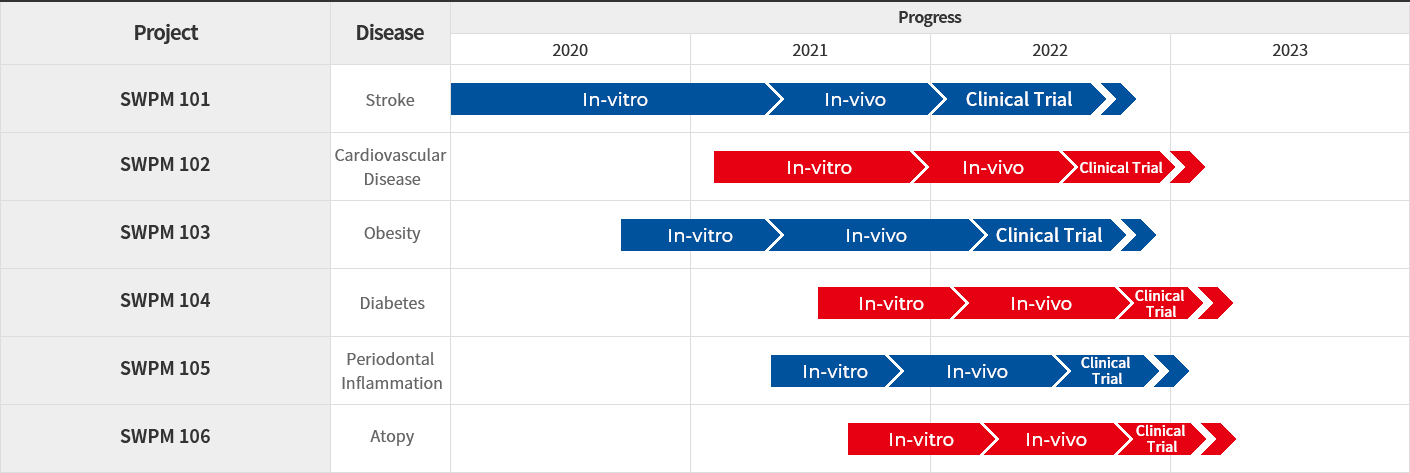
Bio R&D Center has microbiome pipelines for various diseases such as ischemic stroke, periodontitis, Alzheimer's, bacterial vaginitis, and atopy, and is conducting R&D, which aims to develop new drugs.
-
One Team of Wide Experience and Competence
Researchers at the Sungwun Pharmacopia R&D Center have been constantly experiencing, passionate and challenging experiences
We are continuing to innovate and achieve. We have successfully transferred technology and mass commercial production of a number of raw materials and chemical synthetic products to Indonesia. In the field of microbiome, the development of therapeutic agents for ischemic stroke as an indication has taken a step further to commercialization, such as license-out. In addition, we have filed and registered dozens of patents so far and are focusing on the development of innovative products and application technologies to prepare for the rapidly changing global pharmaceutical market.
Pipeline
Active Pharmaceutical Ingredients(APIs) Pipeline
| No. |
API Name |
Indication |
Progress |
| 1 |
Erdosteine |
Expectorant |
Under development |
| 2 |
Clopidogrel Bisulfate |
Platelet Cohesion Suppression |
Under development |
| 3 |
Rabeprazole |
PPI(Proton Pump Inhabitor) |
Under development |
| 4 |
CNU |
Cholesterol Gallbladder Disease |
Under development |
| 5 |
Nafamostat |
Pancreatic Inflammation |
Under development |
| 6 |
Mirodenafil |
Prostate Hypertrophy |
Under development |
| 7 |
Peramivir |
Antiviral Drugs |
Under development |
| 8 |
Lubiprostone |
Irritable Bowel Syndrome |
Under development |
| 9 |
R-(+)-Dexlansoprazole |
Esophagitis |
Under development |
| 10 |
Edoxaban |
Venous Thromboembolism |
Under development |
| 11 |
Nintedanib |
Pulmonary Fibrosis |
Under development |
| 12 |
Tofacitinib |
Hair Loss |
Under development |
Under Development
Technology
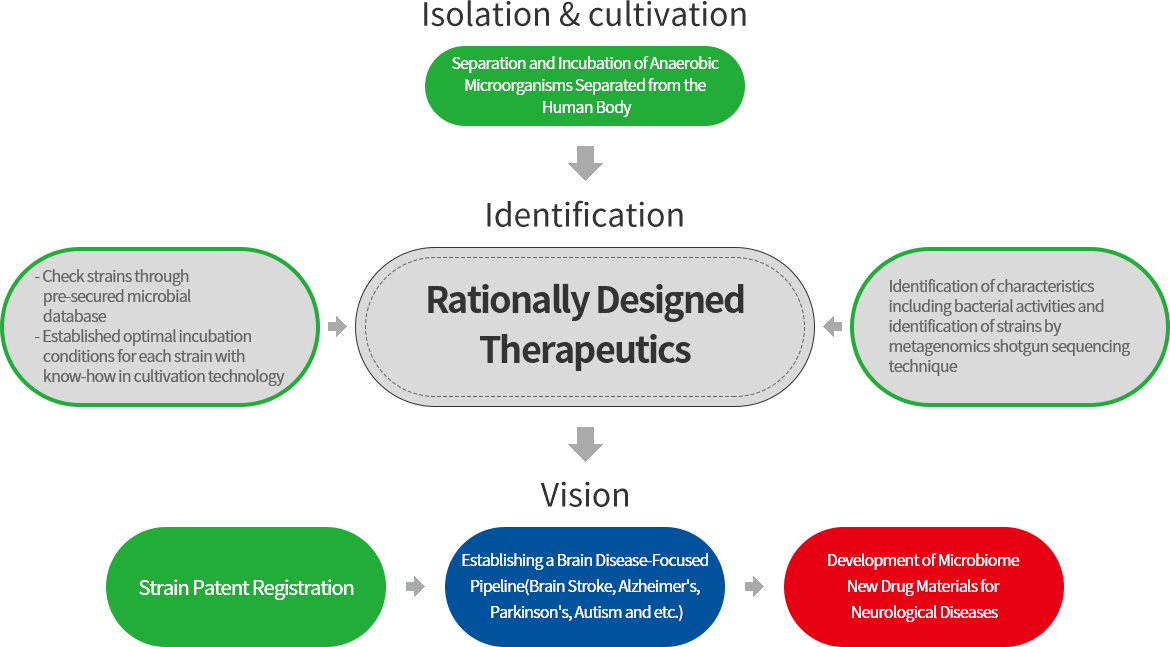
Microbiome Research & Development
 zoom in
zoom in
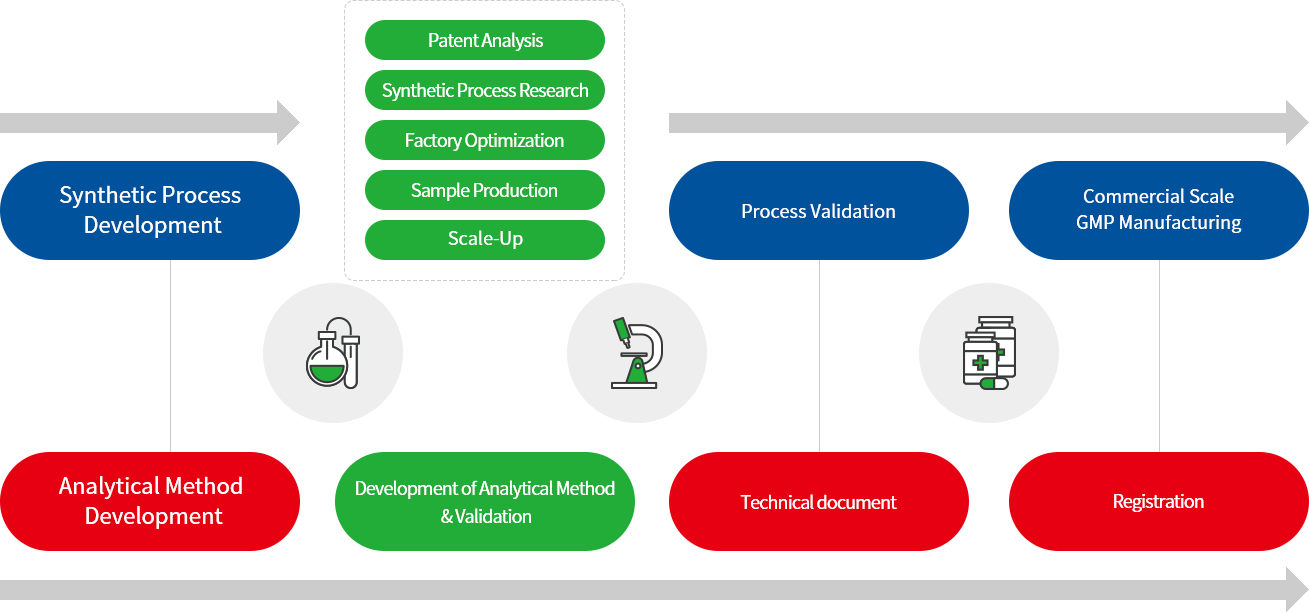
Chemical APIs Research & Development
 zoom in
zoom in